ANSIASQZSampling Procedures and Tables for Inspection by Attributes- ANSI/ASQ Z Sampling Procedures and Tables for Inspection by. This e-standard is a very minor revision of ANSI/ASQ Z (R), also referred to as ANSI/ASQ Z ANSI/ASQ Z Sampling Procedures and Tables for Inspection By. Attributes The FDA recognizes ANSI/ASQ Z as a General consensus standard.
| Author: | Vojar Arashura |
| Country: | Switzerland |
| Language: | English (Spanish) |
| Genre: | Spiritual |
| Published (Last): | 3 September 2017 |
| Pages: | 108 |
| PDF File Size: | 7.66 Mb |
| ePub File Size: | 12.19 Mb |
| ISBN: | 207-6-89056-458-2 |
| Downloads: | 81156 |
| Price: | Free* [*Free Regsitration Required] |
| Uploader: | Vudodal |
Oct 30, 2019 Ansi Asq Z1.4 2008 Pdf Jan 04, 2008 Provided by IHS under license with ASQ No reproduction or networking permitted without license from IHS Not for Resale ANSI/ASQ Z1.4-2008 can be selected by choosing a Limiting Quality (LQ) and a with the AQL and Inspection Level specified for the inspec- consumers risk to be associated with it. . ANSI/ASQ Z1.4 – Current version is ANSI/ASQ Z1.4: 2008. FDA Recognition – The FDA recognizes ANSI/ASQ Z1.4-2008 as a General consensus standard – Extent of Recognition: Use of all Single, Double and Multiple sampling plans according to the standard's switching rules to make acceptance/rejection. ANSI ASQ Z1.4-2008 PDF - ANSIASQZSampling Procedures and Tables for Inspection by Attributes- ANSI/ASQ Z Sampling Procedures and Tables for Inspection. This e-standard is a.
Z1.4:2008 inspection levels
For individual lots with percents nonconforming or nonconformities per units equal to the speci? This standard is not included in any packages. Thus if we claim that we accept zero defects and test a very small sample, in this case five samples, there is a high probability that we are accepting defects in the lot without being able to detect them.
An AQL for a group of nonconformities may be designated in addition to AQLs for individual nonconformities, or subgroups, within that group. Or does it remain at units? Lots or batches found unacceptable shall be resubmitted axq reinspection only after all units are re-examined or re-tested and all nonconforming units are removed or nonconformities corrected.
Inspection by attributes is inspection whereby either the unit of product is classi? Scheme performance is de?
This standard is intended to be used as a system employing tightened, normal, and reduced inspection on a continuing series of lots to achieve consumer protection while assuring the producer that acceptance will occur most of the time if quality is better than the AQL.
ANSI-ASQ Z Sampling Procedures and Tables for Inspection 按属性检查用取样程序_图文_百度文库
As necessary, the supplier shall provide adequate and suitable storage space for each lot or batch, equipment needed for proper identi? Particularly with respect to microbial testing the number of samples are much lower. Ans number of sample units inspected shall be equal to the sample size given by the z1.4-20008.
I am looking to achieve a The standard divides inspection levels into two main categories: Incorrect anai can result in regulatory observations. The inspection level determines the relationship between the lot or batch size and the sample size. The lot or batch size is the number of units of product in a lot or batch. The reduced inspection can be used conditionally when the normal inspection passes for more than two consecutive lots.
Note, the sampling plan consists assq a sample size and acceptance criteria at particular AQL. Binomial distribution used for percent nonconforming computations; Poisson for nonconformities per hundred units.
The following two de? The curves assume no curtailment of inspection and are approximate to the extent that they z14.-2008 based upon the Poisson distribution, and that the sample sizes at each stage for double and multiple sampling are assumed to be 0.
They show the average sample size for scheme performance when using single sampling. The standard is intended for inspection of final product, components and raw materials, materials in process, and data and records.
Different AQLs may be designated for different types as defects critical, major, and minor. Click here to sign up. If sample size equals, or exceeds, lot size, carry out percent inspection.
The ideal method of calculating the sample size and risk is by use of the hypergeometric probability function. According to the standard, inspection Level II should be used unless otherwise specified.
Tag: Z1.4:2008
Another consideration is the percentage of contaminated units per lot. Is there a practical or common sense procedure to follow? Usually the administrative dif?
Defect categories are divided based on criticality to product quality attributes. Another approach is using light to illuminate the contamination, such as a black light UVA. For instance, if inspecting a bottle for tablet count, closure, seal, label and carton defects, these defects are not added together since they are results of different packaging processes. Although complicated, ini resample the lot.
Most Related
This standard is a revision of ANSI/ASQC Z,. “Sampling Procedures and Tables for Inspection by. Attributes.” Beyond editorial refinements, only the. Know the switching rules for ANSI/ASQ Z Categorize the various sampling plan systems in terms of lot-by-lot, continuous production, attributes or variables. ANSI/ASQ Z Sampling Procedures and Tables for Inspection By. Attributes The FDA recognizes ANSI/ASQ Z as a General consensus standard.
| Author: | Daisida Kigazuru |
| Country: | Turkmenistan |
| Language: | English (Spanish) |
| Genre: | Photos |
| Published (Last): | 21 June 2007 |
| Pages: | 318 |
| PDF File Size: | 9.90 Mb |
| ePub File Size: | 4.63 Mb |
| ISBN: | 124-2-72622-826-4 |
| Downloads: | 85871 |
| Price: | Free* [*Free Regsitration Required] |
| Uploader: | Brara |
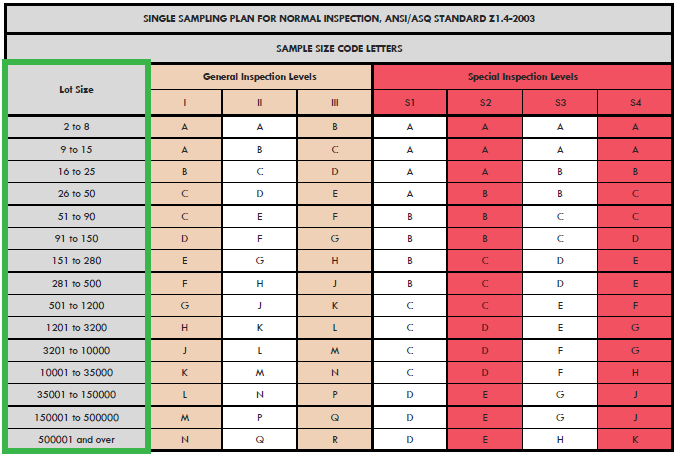
General Inspection Level II, Normal, shows that for a lot size of 20, a sample size code level of M corresponds to a sample size of aeqc As the voice of the U. Another consideration is the percentage of contaminated units per lot. Use double sampling plan above or alternatively use code letter D.
ANSI/ASQ Z– (R): Sampling Procedures and Tables for Inspection by Attributes | ASQ
I hope this helps. Under multiple sampling, the procedure shall be similar to that speci?
Discontinue Inspection Under Zz1.4-2008. Values given in the table above are based on the Poisson distribution as an approximation to the binomial distribution See The results will be used to identify enhancement opportunities to our database and identifying improvements to the current and more frequent processes.

These plans are intended primarily to be used for a continuing series of lots or batches. Table X-D—Tables for sample size code letter: The team will use a system form in Metastorm to capture activities throughout the day.
ANSI-ASQ Z Sampling Procedures and Tables for Inspection 按属性检查用取样程序_图文_百度文库
Ansi Asq Z1.4 2008 Pdf Full
Table I shall be used to? The supplier may be required at the discretion of the responsible authority to inspect every unit of the lot or batch for designated classes of nonconformities. An American national Standard implies a consensus of those zsqc concerned with its scope and provisions.
In general, the function of such classi? The operating characteristic curves of scheme performance are based on the use of limit numbers in 1z.4-2008 to reduced inspection and are approximately correct when the limit numbers for reduced inspection are not used under Option 8.
ANSI/ASQ Z1.4–2003 (R2013): Sampling Procedures and Tables for Inspection by Attributes
By smaller, it is less than 1 standard deviation from the data that has been detected. Since you have no previous data and you are getting 5 samples an hour from each employee, assuming a 7 hour workday, taking out lunch and two breaksthat will give you approximately 35 samples a day.
The responsible authority shall determine whether normal or tightened inspection shall be used on reinspection and whether reinspection shall include all types or classes of nonconformities or only the particular types or classes of nonconformities which caused initial rejection.
Particularly with respect to microbial testing the number of samples are much lower. It may be a single article, a pair, a set, a length, an area, an operation, a volume, a component of an end product, or the end product itself. Now, you will probably want to extend this out another three weeks so that you have an idea of what happens over a month. They show the average outgoing quality limits for scheme performance when using single sampling.
Ansi Asq Z1 4 2008 Pdf
Four additional special levels: Steven Walfish Secretary, U. These rules are designed to encourage suppliers to have process averages consistently better than the AQL.
D Acceptance Quality Limits normal inspection 1. How can we improve our incoming inspection process? However, we run a variety of tests, including microbial and heavy metal testing. Rejection in an acceptance sampling sense means to decide that a batch, lot or quantity of product, material, or service has not been shown to satisfy the acceptance criteria based on the information obtained from the sample s.
The extent of nonconformance of product shall be expressed either in terms of percent nonconforming or in terms of nonconformities per hundred units.
Tag: Z1.4:2008
Ansi Asq Z1 4 2008 Pdf
If that is not possible or practical, then percent inspection using a quick, inexpensive, and effective method permits you to avoid uncertainties with sampling. These tests are very costly. Normal, tightened or reduced inspection shall continue unchanged on successive lots or batches except where the switching procedures given below require change. The operating characteristic curve for unquali?
An AQL for a group of nonconformities may be designated in addition to AQLs for individual nonconformities, or subgroups, aqc that group. Tables VI and VII give process levels for which the probabilities of lot acceptance under various sampling plans are 10 percent and z1.4-20008 percent respectively. If the cumulative number of lots not accepted in a sequence of consecutive lots on tightened inspection reaches 5, the acceptance procedures of this standard shall be discontinued.
Norma Ansi Asq Z1 4 2008 Pdf
A sample consists of one or more units of product drawn from a lot or batch, the units of the sample being selected at random without regard to their quality.
The following two de? Can I get further explanation of how one would justify that less discrimination is needed? Apparently, you are able detect the container contamination prior to filling them, or are able to andi the effect of the contamination on the final product. Table X-E—Tables for sample size code letter: